|
Getting your Trinity Audio player ready...
|

Nitrosamines are chemical compounds that can be present as contaminants in various products, including foods (such as certain beverages), tobacco, rubber and cosmetics. Some of these nitrosamines, such as N-nitrosodiethanolamine (NDELA) and N-nitrosodimethylamine (NDMA), are classified as category 1B carcinogens. Cosmetic products containing nitrosamines, including NDELA, are prohibited by European cosmetic regulation (EC) No. 1223/2009, and its Annex III refers to the limit of 50 μg/kg that may be present in cosmetic ingredients, which is associated at MoS (margin of safety) >> 10,000, using BMDL10 (a reference dose associated with 10% additional background-adjusted tumor risk) at 0.73 mg/kg bw/d as starting point (PoD). For some substances, such as mono-, di-, trialkylamines, alkanolamines or specific hair dyes (ethanolamine derivatives), specific purity criteria have been defined regarding the nitrosamine content (Annex III). Other (non-regulated) materials, depending on their structural composition and manufacturing technology, may contain traces of nitrosamines (SCCS/1486/12).
The formation of N-nitrosamines in cosmetic products may result from the confluence of three factors: i) the presence of precursor ingredients with structures that are capable of undergoing nitrosation (i.e., nitrosable structures), ii) the availability of a nitrosating agent, and iii) the existence of suitable reaction conditions. The occurrence of N-nitrosation is conditional, depending on both the nitrosating agent and the substrate, and can occur in acidic, neutral or alkaline conditions.
Although N-nitrosamines can be formed from primary, secondary and tertiary amines, the formation of N-nitrosamines is more favored with secondary amines under conditions of manufacture, storage or use. For amines with different degrees of substitution (i.e. non-secondary), the probability of N-nitrosation is much lower. Attention in cosmetic products should be mainly focused on secondary amines, since the resulting N-nitrosamines are the most stable and likely to result in consumer exposure. Primary amines do not produce stable N-nitrosamines. These can easily interact with nitrosating agents, leading to the formation of unstable N-nitroso compounds, but they decompose quickly, resulting in diazonium salts instead of producing N-nitrosamines. Nitrosation of most tertiary amines occurs at a remarkably slow rate and is generally not a concern for cosmetics.
Nitrosamines are compounds containing the functional group R1R2N-N=O. Nitrosation is the process of converting organic compounds (e.g., alkyl and arylamines and amides) into nitrous derivatives (e.g., nitrosamines and nitrosamides) by reaction with nitrosating agents. These agents include nitrous acid (HNO2), nitrogen oxides (e.g., nitrites, nitrates, and dinitrogen trioxide), and other compounds capable of generating a nitrosonium ion, NO+ (Figure 1).
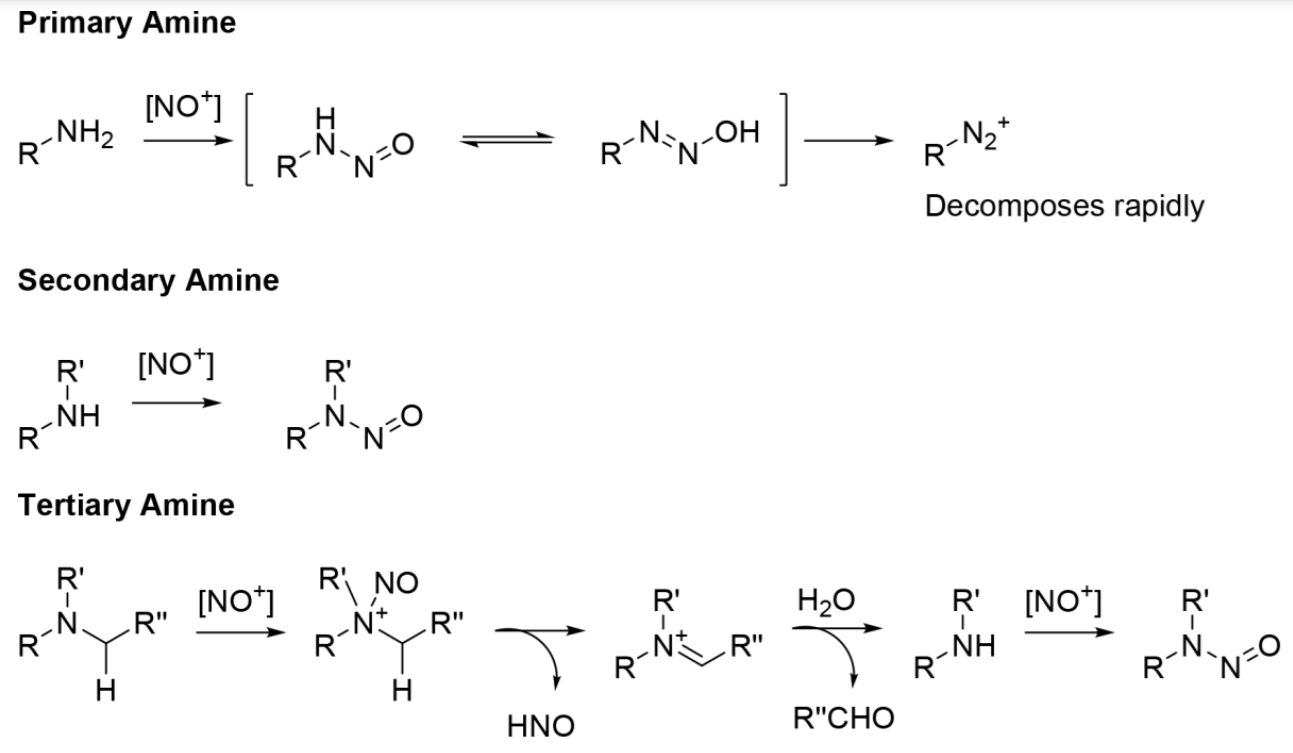
In relation to cosmetics, the major concern is the conversion of secondary amines (R1-NH-R2) into N-nitrosamines, which can be carcinogenic. Of the approximately 300 N-nitroso compounds that were tested, 85% of the 209 nitrosamines and 92% of the 86 nitrosamides were shown to produce cancer in laboratory animals. Nitrosation can occur under physiological conditions. Depending on the nitrosating agent and substrate, nitrosation can occur under acidic, neutral or alkaline conditions. However, nitrosation most commonly occurs under acidic conditions. Atmospheric or physiological NO2 can also participate in the nitrosation of amines in aqueous solution.
Another concern is when nitrosamines may be present in a cosmetic as an impurity in an ingredient. This concern became apparent during the safety assessment of morpholine (08/1989), where the Expert Panel for the Safety of Cosmetic Ingredients (CIR Expert Panel) determined that, under conditions of cosmetic use, it is highly unlikely that morpholine will be completely free of carcinogenic N-nitrosamines. Nitrosation of morpholine to form N-nitrosomorpholine occurs readily. Consequently, concern has been raised about contamination of morpholine with N-nitrosomorphine. Although amines (they exist as impurities or decomposition products of raw materials) may not be mutagenic or carcinogenic by themselves, in the presence of a nitrosating agent they may have mutagenic and carcinogenic potential, due to the reactions mentioned above. Although many secondary amines are readily nitrosated to form isolatable N-nitrosamines and N-nitrosamides, the primary alkyl and arylamines ultimately produce diazonium salts rather than nitrosamines (Figure 1). Tertiary alkylamines also do not tend to react with nitrosating agents to form nitrosamines. Although tertiary arylamines undergo nitrosation, the reaction occurs at the aromatic ring and does not result in the formation of nitrosamines.
The industry guide published by the US Food & Drug Administration (FDA) on 04/08/2023 provides the recommended acceptable limits for nitrosamine impurities in medicines (NDSRIs Guidance, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/updated-information-recommended-acceptable-intake-limits-nitrosamine-drug-substance-related) to predict the mutagenic and carcinogenic potential of a drug.
To assist manufacturers and applicants in conducting safety testing, the FDA recommends that if in vitro mutagenicity testing is contemplated, an enhanced Ames assay be used to evaluate whether an NDSRI poses a mutagenic risk. A negative result in a valid enhanced Ames assay can be used to support a higher threshold for an NDSRI; however, manufacturers and applicants should note that the FDA may request additional safety data, beyond the enhanced Ames assay, to support alternative AI thresholds. The following recommendations represent FDA's current thinking; Data gaps in the information available to support the safety of NDSRIs continue to be addressed in future research. If a standard Ames assay (OECD TG No. 471, “Bacterial Reverse Mutation Test”) is performed on an NDSRI and produces a positive result, the FDA recommends that there is no need to perform an additional assay using enhanced test conditions.
In Brazil, RDC 283/2019 establishes rules for the investigation, control and elimination of nitrosamines in medicines and pharmaceutical ingredients. The regulatory measure applies to companies that manufacture, import and fractionate these products. Furthermore, ANVISA published Guide Nº50/2021 (version 3) on the Control of Nitrosamines in Active Pharmaceutical Ingredients and Medicines (effective from 06/13/2023). This guide presents basic concepts about training, risk management related to the presence, as well as recommendations on the control of nitrosamines in active pharmaceutical ingredients and medicines, as well as clarifying the responsibility of companies, and presents strategies for calculating limits and addresses other concepts .
The link below contains specific comments on the draft reviewed by the CIR Expert Panel between 04 and 05/12/2023.
https://www.cir-safety.org/sites/default/files/ADMIN_NitrosationDoc.pdf
Want to know more about Security Assessment?
- Continue browsing our website.
- Follow our social media pages on Facebook and Instagram.
Safety in cosmetics. Because you need to have it.
SF Safety Consulting.
Passion for knowledge and science.
